Covalent Bonding – Double Bonds
This page explains how double covalent bonds arise. It starts with a simple picture of double covalent bonding, and then takes a more sophisticated view of the bonding in ethene.
Warning! This page assumes that you have already read the page on single covalent bonds.
A Simple View of Double Covalent Bonds
A double covalent bond is where two pairs of electrons are shared between the atoms rather than just one pair.
Some Simple Molecules Containing Double Bonds
Oxygen, O2
Two oxygen atoms can both achieve stable structures by sharing two pairs of electrons as in the diagram.
The double bond is shown conventionally by two lines joining the atoms. Each line represents one pair of shared electrons.
Carbon dioxide, CO2
Ethene, C2H4
Ethene has a double bond between the two carbon atoms.
A More Sophisticated View of the Bonding in Ethene
It is important to explore the bonding in ethene in more detail because it has a direct impact on its chemistry. Unless you have some understanding of the true nature of the double bond, you can't really understand the way that ethene behaves.
An Orbital View of the Bonding in Ethene
Ethene is built from hydrogen atoms (1s1) and carbon atoms (1s22s22px12py1).
Promotion of an electron
The carbon atom doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s2 pair into the empty 2pz orbital. This is exactly the same as happens whenever carbon forms bonds – whatever else it ends up joined to.
The carbon atom is now in an excited state.
Hybridisation
In the case of ethene, there is a difference from methane because each carbon is only joining to three other atoms rather than four. When the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise three of the orbitals rather than all four. They use the 2s electron and two of the 2p electrons, but leave the other 2p electron unchanged.
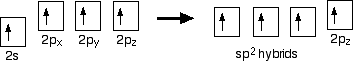
Note: You might wonder why it chooses to hybridise these three orbitals rather than just use the three p orbitals which already have the same energy. It's because it uses the orbitals with the lowest energy first.
The new orbitals formed are called sp2 hybrids, because they are made by an s orbital and two p orbitals reorganising themselves. sp2 orbitals look rather like sp3 orbitals that we discussed in the bonding in methane in the page on single bonds, except that they are shorter and fatter. The three sp2 hybrid orbitals arrange themselves as far apart as possible – which is at 120° to each other in a plane. The remaining p orbital is at right angles to them.
The two carbon atoms and four hydrogen atoms would look like this before they joined together:
The various atomic orbitals which are pointing towards each other now merge to give molecular orbitals, each containing a bonding pair of electrons. Molecular orbitals made by end-to-end overlap of atomic orbitals are called sigma bonds.
The p orbitals on each carbon aren't pointing towards each other, and so we'll leave those for a moment. In the diagram, the black dots represent the nuclei of the atoms.
Notice that the p orbitals are so close that they are overlapping sideways.
This sideways overlap also creates a molecular orbital, but of a different kind. In this one the electrons aren't held on the line between the two nuclei, but above and below the plane of the molecule. A bond formed in this way is called a π bond.
For clarity, the sigma bonds are shown using lines – each line representing one pair of shared electrons. The various sorts of line show the directions the bonds point in. An ordinary line represents a bond in the plane of the screen (or the paper if you've printed it), a broken line is a bond going back away from you, and a wedge shows a bond coming out towards you.
Note: The really interesting bond in ethene is the π bond. In almost all cases where you will draw the structure of ethene, the sigma bonds will be shown as lines.
Be clear about what a π bond is. It is a region of space in which you can find the two electrons which make up the bond. Those two electrons can live anywhere within that space. It would be quite misleading to think of one living in the top and the other in the bottom.
Taking chemistry further: This is a good example of the curious behaviour of electrons. How do the electrons get from one half of the π bond to the other if they are never found in between? It's an unanswerable question if you think of electrons as particles. If you want to follow this up, you will have to read some fairly high-powered stuff on the wave nature of electrons.
Even if your syllabus doesn't expect you to know how a π bond is formed, it will expect you to know that it exists. The π bond dominates the chemistry of ethene. It is very vulnerable to attack – a very negative region of space above and below the plane of the molecule. It is also somewhat distant from the control of the nuclei and so is a weaker bond than the sigma bond joining the two carbons.
Important! Check your syllabus! Find out whether you actually need to know how a π bond is formed. Don't forget to look under ethene as well as in the bonding section of your syllabus. If you don't need to know it, there's no point in learning it! You will, however, need to know that a π bond exists – that the two bonds between the carbon atoms in ethene aren't both the same. If you are working to a UK-based syllabus, but haven't got a copy of it, find out how to download one.
All double bonds (whatever atoms they might be joining) will consist of a sigma bond and a π bond.
This orbital view of the double bond is only really important at this level with regard to organic compounds. If you want to read more about this, follow the first link below which leads you to the menu for a section specifically on organic bonding. You will find the description of ethene repeated, but will also find information about the bonding in benzene and in the carbon-oxygen double bond.
Where would you like to go now?
To explore more organic bonding To the bonding menu To the atomic structure and bonding menu To Main Menu