Reactions of Acid Anhydrides With Water, Alcohols or Phenol
This page looks at the reactions of acid anhydrides with water, alcohols and phenols (including the manufacture of aspirin). These reactions are all considered together because their chemistry is so similar.
There is also a great similarity between acid anhydrides and acyl chlorides (acid chlorides) as far as these reactions are concerned. Concentrate on these similarities as you go through this page because it should help you to remember.
Similarities Between the Reactions
Comparing the structures of water, ethanol and phenol
Each substance contains an -OH group. In water, this is attached to a hydrogen atom. In an alcohol, it is attached to an alkyl group – shown in the diagrams below as "R". In phenols, it is attached to a benzene ring. Phenol (the simplest member of the family of phenols) is C6H5OH.
Note: If you aren't sure about using this symbol for a benzene ring, you could follow this link to find out all about it. It is likely to take you some time, though, and you may have to visit several other pages as well.
It isn't particularly important in the context of the current page. All you need to know is that at each corner of the hexagon there is a carbon atom, together with a hydrogen atom apart from where the -OH group is attached.
Comparing the Reactions of Acyl Chlorides and Acid Anhydrides With These Compounds
Because the formula is much easier, it helps to start with the acyl chlorides.
The reactions with acyl chlorides
We'll take ethanoyl chloride as typical of the acyl chlorides.
Taking a general case of a reaction between ethanoyl chloride and a compound X-O-H (where X is hydrogen, or an alkyl group or a benzene ring):
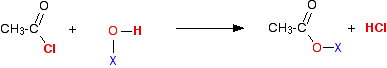
So in each case, hydrogen chloride gas is produced – the hydrogen coming from the -OH group, and the chlorine from the ethanoyl chloride. Everything left over just gets joined together.
The same reactions with acid anhydrides
Ethanoic anhydride is the only one you are likely to come across for UK A-level purposes.
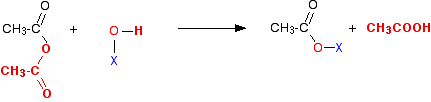
If you compare this with the acyl chloride equation, you can see that the only difference is that ethanoic acid is produced as the second product of the reaction rather than hydrogen chloride.
Note: The colour coding in these equations is to try to help you to see where everything ends up and where it came from, and to enable you to compare the two reactions more easily. You can think of the entire bit of the ethanoic anhydride shown in red as being exactly the equivalent of the chlorine atom in the acyl chloride as far as these reactions are concerned.
These reactions are just the same as the corresponding acyl chloride reactions except:
- Ethanoic acid is formed as the second product rather than hydrogen chloride gas.
- The reactions are slower. Acid anhydrides aren't so violently reactive as acyl chlorides.
The Individual Reactions
The Reaction With Water
Modifying the general equation we've just looked at, you will see that you just get two molecules of ethanoic acid produced.
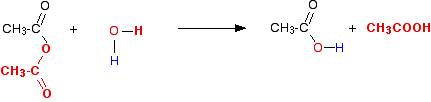
This is more usually (and more easily!) written as:
The reaction happens slowly at room temperature (faster on gentle warming) without a great deal exciting to observe – unlike in the acyl chloride case where hydrogen chloride fumes are produced. You mix two colourless liquids and get another colourless liquid!
The equivalent acyl chloride reaction is:
The Reaction With Alcohols
We'll start by taking the general case of any alcohol reacting with ethanoic anhydride. The equation would be:
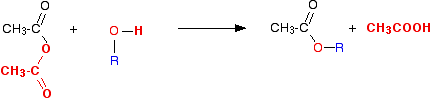
or, more simply:
The product this time (apart from the ethanoic acid always produced) is an ester. For example, with ethanol you would get the ester ethyl ethanoate:
This reaction also needs gentle heating for it to happen at a reasonable rate, and again there isn't anything visually dramatic.
The equivalent acyl chloride reaction is:
Note: If you are exceptionally wide awake, you might wonder why the reaction between ethanoic anhydride and an alcohol doesn't result in two molecules of ester. Carboxylic acids like ethanoic acid react with alcohols to give esters – so why doesn't this happen with the acid molecule that is formed reacting with alcohol in the mixture?
The reason is simply one of reaction conditions. This reaction is happening in the absence of any catalysts. To get carboxylic acids and alcohols to react at any sort of reasonable rate, you need heat and a catalyst such as concentrated sulfuric acid.
The Reaction With Phenols
The reaction with phenol itself
Phenols have an -OH group attached directly to a benzene ring. In the substance normally called "phenol", there isn't anything else attached to the ring as well. We'll look at that first.
The reaction between phenol and ethanoic anhydride isn't particularly important, but you would get an ester just as you do with an alcohol.
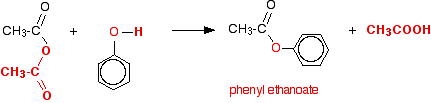
Or, more simply:
Especially if you write the equation in this second way, it is obvious that you have just produced another ester – in this case, called phenyl ethanoate.
The equivalent acyl chloride reaction is:
But beware! You may come across the structure of the ester drawn in a variety of other ways which make it look much more as if it was a derivative of phenol (which of course it is!).
For example:

Looking at it this way, notice that the hydrogen of the phenol -OH group has been replaced by an acyl group – an alkyl group attached to a carbon-oxygen double bond.
You can say that the phenol has been acylated or has undergone acylation.
Because of the nature of this particular acyl group, it is also described as ethanoylation. The hydrogen is being replaced by an ethanoyl group, CH3CO-.
Using a similar reaction to make aspirin
The reaction with phenol itself isn't very important, but you can make aspirin by a very similar reaction.
The molecule below is 2-hydroxybenzoic acid (also known as 2-hydroxybenzenecarboxylic acid). The old name for this is salicylic acid.
You might find it written in either of these two ways. They are the same structure with the molecule just flipped over in space.
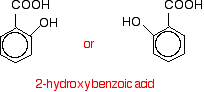
You might also find it with the -OH group at the top and the -COOH group next door and either to the left or right of it. Life can get very confusing!
When this reacts with ethanoic anhydride, it is ethanoylated (or acylated, if you want to use the more general term) to give:
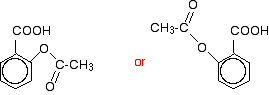
You might find all sorts of other variants on drawing this as well.
This molecule is aspirin.
Although this reaction can also be done with ethanoyl chloride, aspirin is manufactured by reacting 2-hydroxybenzoic acid with ethanoic anhydride at 90°C.
The reasons for using ethanoic anhydride rather than ethanoyl chloride include:
- Ethanoic anhydride is cheaper than ethanoyl chloride.
- Ethanoic anhydride is safer to use than ethanoyl chloride. It is less corrosive and not so readily hydrolysed (its reaction with water is slower).
- Ethanoic anhydride doesn't produce dangerous (corrosive and poisonous) fumes of hydrogen chloride.
Questions to test your understanding
Questions on the reaction of acid anhydrides with water, alcohols and phenols AnswersWhere would you like to go now?
To the acid anhydrides menu To the menu of other organic compounds To Main Menu