Reactions of Acyl Chlorides With Ammonia or Primary Amines
This page looks at the reactions of acyl chlorides (acid chlorides) with ammonia and with primary amines. These reactions are considered together because their chemistry is so similar.
Similarities Between the Reactions
Comparing the Structures of Ammonia and Primary Amines
Each substance contains an –NH2 group. In ammonia, this is attached to a hydrogen atom. In a primary amine, it is attached to an alkyl group (shown by "R" in the diagram below) or a benzene ring.
Note: If you aren't sure about using this symbol for a benzene ring, you could follow this link to find out all about it. It is likely to take you some time, though, and you may have to visit several other pages as well.
It isn't particularly important in the context of the current page. All you need to know is that at each corner of the hexagon there is a carbon atom, together with a hydrogen atom apart from where the -NH2 group is attached.
If you want to know more about what primary amines are, you could follow this link to a page introducing amines, and just read the beginning of it.
What Happens When These React With an Acyl Chloride?
We'll take ethanoyl chloride as typical of the acyl chlorides. For UK A-level purposes, it is the one you are most likely to be asked about anyway.
Taking a general case of a reaction between ethanoyl chloride and a compound XNH2 (where X is hydrogen, or an alkyl group, or a benzene ring):
The reaction happens in two stages.
First:
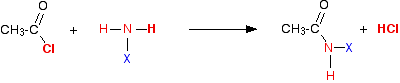
So in each case, you initially get hydrogen chloride gas – the hydrogen coming from the -NH2 group, and the chlorine from the ethanoyl chloride. Everything left over just gets joined together.
But ammonia and amines are basic, and react with the hydrogen chloride to produce a salt. So the second stage of the reaction is:
This is easier to understand with real compounds – as you will see below.
Note: If you are interested in exploring the general mechanism for these reactions, you will find it by following this link to another part of the site dealing with nucleophilic addition-elimination reactions.
The Individual Reactions
The Reaction with Ammonia
In this case, the "X" in the equations above is a hydrogen atom. So in the first instance you get hydrogen chloride gas and an organic compound called an amide.
Amides contain the group -CONH2. In the reaction between ethanoyl chloride and ammonia, the amide formed is called ethanamide.
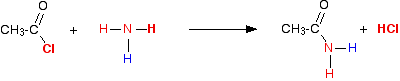
This is more usually (and more easily!) written as:
The hydrogen chloride produced reacts with excess ammonia to give ammonium chloride.
and you can combine all this together to give one overall equation:
You normally add the ethanoyl chloride to a concentrated solution of ammonia in water. There is a very violent reaction producing lots of white smoke – a mixture of solid ammonium chloride and ethanamide. Some of the mixture remains dissolved in water as a colourless solution.
Note: If you want the mechanism for this reaction, you will find it by following this link to another part of the site dealing with nucleophilic addition-elimination reactions.
The Reaction With Primary Amines
The reaction with methylamine
We'll take methylamine as typical of primary amines where the -NH2 is attached to an alkyl group.
The initial equation would be:
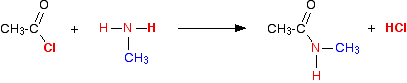
The organic product this time is called an N-substituted amide.
If you compare the structure with the amide produced in the reaction with ammonia, the only difference is that one of the hydrogens on the nitrogen has been substituted for a methyl group.
This particular compound is N-methylethanamide. The "N" simply shows that the substitution is on the nitrogen atom, and not elsewhere in the molecule.
The equation would normally be written:
You can think of primary amines as just being modified ammonia. If ammonia is basic and forms a salt with the hydrogen chloride, excess methylamine will do exactly the same thing.
The salt is called methylammonium chloride. It is just like ammonium chloride, except that one of the hydrogens has been replaced by a methyl group.
You would usually combine these equations into one overall equation for the reaction:
Note: If you are want the mechanism for this reaction (although using ethylamine rather than methylamine as the example), you will find it by following this link to another part of the site dealing with nucleophilic addition-elimination reactions.
The reaction looks exactly the same as the one with ammonia. The methylamine is again used as a concentrated solution in water. There is a violent reaction producing a white solid mixture of N-methylethanamide and methylammonium chloride.
The reaction with phenylamine (aniline)
Phenylamine is the simplest primary amine where the -NH2 group is attached directly to a benzene ring. Its old name is aniline.
In phenylamine, there isn't anything else attached to the ring as well. You can write the formula of phenylamine as C6H5NH2.
There is no essential difference between this reaction and the reaction with methylamine, except that the phenylamine is a brownish liquid, and the solid products tend to be stained brownish.
The overall equation for the reaction is:
The products are N-phenylethanamide and phenylammonium chloride.
This reaction can sometimes look confusing if the phenylamine is drawn showing the benzene ring, and especially if the reaction is looked at from the point of view of the phenylamine.
For example, the product molecule might be drawn looking like this:
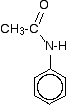
If you stop and think about it, this is obviously the same molecule as in the equation above, but it stresses the phenylamine part of it much more.
Looking at it this way, notice that one of the hydrogens of the -NH2 group has been replaced by an acyl group – an alkyl group attached to a carbon-oxygen double bond.
You can say that the phenylamine has been acylated or has undergone acylation.
Because of the nature of this particular acyl group, it is also described as ethanoylation. The hydrogen is being replaced by an ethanoyl group, CH3CO-.
Questions to test your understanding
Questions on the reactions of acyl chlorides with ammonia and primary amines AnswersWhere would you like to go now?
To the acyl chlorides menu To the menu of other organic compounds To Main Menu