Nucleophilic Substitution Reactions of Halogenoalkanes and Hydroxide Ions
This page gives you the facts and simple, uncluttered mechanisms for the nucleophilic substitution reactions between halogenoalkanes and hydroxide ions (from, for example, sodium hydroxide). If you want the mechanisms explained to you in detail, there is a link at the bottom of the page.
The Reaction of Primary Halogenoalkanes With Hydroxide Ions
Important! If you aren't sure about the difference between primary, secondary and tertiary halogenoalkanes, it is essential that you follow this link before you go on.
The Facts
If a halogenoalkane is heated under reflux with a solution of sodium or potassium hydroxide, the halogen is replaced by -OH and an alcohol is produced. Heating under reflux means heating with a condenser placed vertically in the flask to prevent loss of volatile substances from the mixture.
The solvent is usually a 50/50 mixture of ethanol and water, because everything will dissolve in that. The halogenoalkane is insoluble in water. If you used water alone as the solvent, the halogenoalkane and the sodium hydroxide solution wouldn't mix and the reaction could only happen where the two layers met.
For example, using 1-bromopropane as a typical primary halogenoalkane:
You could write the full equation rather than the ionic one, but it slightly obscures what's going on:
The bromine (or other halogen) in the halogenoalkane is simply replaced by an -OH group – hence a substitution reaction. In this example, propan-1-ol is formed.
The Mechanism
Here is the mechanism for the reaction involving bromoethane:
This is an example of nucleophilic substitution.
Because the mechanism involves collision between two species in the slow step (in this case, the only step) of the reaction, it is known as an SN2 reaction.
Note: Unless your syllabus specifically mentions SN2 by name, you can just call it nucleophilic substitution.
If your examiners want you to show the transition state, draw the mechanism like this:
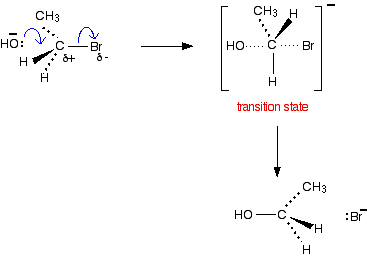
The Reaction of Tertiary Halogenoalkanes With Hydroxide Ions
The Facts
The facts of the reaction are exactly the same as with primary halogenoalkanes. If the halogenoalkane is heated under reflux with a solution of sodium or potassium hydroxide in a mixture of ethanol and water, the halogen is replaced by -OH, and an alcohol is produced.
For example:
Or if you want the full equation rather than the ionic one:
The Mechanism
This mechanism involves an initial ionisation of the halogenoalkane:
followed by a very rapid attack by the hydroxide ion on the carbocation (carbonium ion) formed:
This is again an example ofnucleophilic substitution.
This time the slow step of the reaction only involves one species – the halogenoalkane. It is known as an SN1 reaction.
The Reaction of Secondary Halogenoalkanes With Hydroxide Ions
The Facts
The facts of the reaction are exactly the same as with primary or tertiary halogenoalkanes. The halogenoalkane is heated under reflux with a solution of sodium or potassium hydroxide in a mixture of ethanol and water.
For example:
The Mechanism
Secondary halogenoalkanes use both SN2 and SN1 mechanisms. For example, the SN2 mechanism is:
Should you need it, the two stages of the SN1 mechanism are:
Note: There is another reaction between halogenoalkanes and hydroxide ions involving an elimination reaction. If you want to explore that as an alternative, follow this link.
Where would you like to go now?
Help! Talk me through these mechanisms To menu of nucleophilic substitution reactions To menu of other types of mechanism To Main Menu