Elimination From Unsymmetric Halogenoalkanes
This page looks at elimination from unsymmetric halogenoalkanes such as 2-bromobutane.
2-bromobutane is an unsymmetric halogenoalkane in the sense that it has a CH3 group one side of the C-Br bond and a CH2CH3 group the other.
You have to be careful with compounds like this because of the possibility of more than one elimination product depending on where the hydrogen is removed from.
The basic facts and mechanisms for these reactions are exactly the same as with simple halogenoalkanes like 2-bromopropane. This page only deals with the extra problems created by the possibility of more than one elimination product.
Important! What follows assumes that you are familiar with the mechanism for elimination from 2-bromopropane. If you aren't, it is essential that you follow this link before you go on.
Background to the Mechanism
You will remember that elimination happens when a hydroxide ion (from, for example, sodium hydroxide) acts as a base and removes a hydrogen as a hydrogen ion from the halogenoalkane.
For example, in the simple case of elimination from 2-bromopropane:
The hydroxide ion removes a hydrogen from one of the carbon atoms next door to the carbon-bromine bond, and the various electron shifts then lead to the formation of the alkene – in this case, propene.
With an unsymmetric halogenoalkane like 2-bromobutane, there are several hydrogens which might possibly get removed. You need to think about each of these possibilities.
Where Does the Hydrogen Get Removed From?
The hydrogen has to be removed from a carbon atom adjacent to the carbon-bromine bond. If an OH- ion hit one of the hydrogens on the right-hand CH3 group in the 2-bromobutane (as we've drawn it), there's nowhere for the reaction to go.
To make room for the electron pair to form a double bond between the carbons, you would have to expel a hydrogen from the CH2 group as a hydride ion, H-. That is energetically much too difficult, and so this reaction doesn't happen.
That still leaves the possibility of removing a hydrogen either from the left-hand CH3 or from the CH2 group.
If it was removed from the CH3 group:
The product is but-1-ene, CH2=CHCH2CH3.
If it was removed from the CH2 group:
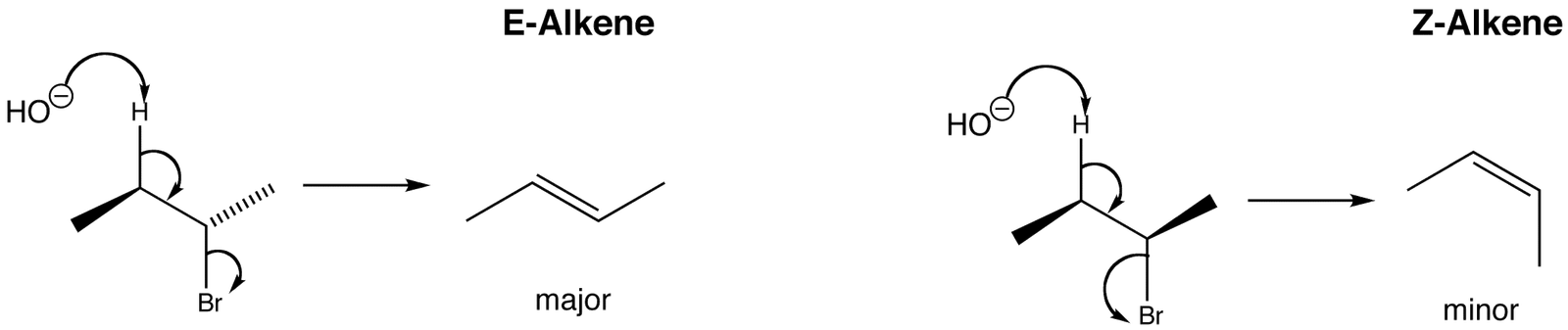
This time the product is but-2-ene, CH3CH=CHCH3.
In fact the situation is even more complicated than it looks, because but-2-ene exhibits geometric isomerism. You get a mixture of two isomers formed – cis-but-2-ene and trans-but-2-ene.
Cis-but-2-ene is also known as (Z)-but-2-ene; trans-but-2-ene is also known as (E)-but-2-ene. For an explanation of the two ways of naming these two compounds, follow the link in the box below.
Which isomer gets formed is just a matter of chance.
Geometric isomerism: Isomerism is where you can draw more than one arrangement of the atoms for a given molecular formula. Geometric isomerism is a special case of this involving molecules which have restricted rotation around one of the bonds – in this case, a carbon-carbon double bond. The C=C bond could only rotate if enough energy is put in to break the π bond. Effectively, except at high temperatures, the C=C bond is "locked".
In the case of but-2-ene, the two CH3 groups will either both be locked on one side of the C=C (to give the cis or (Z) isomer), or on opposite sides (to give the trans or (E) one).
For a full discussion of geometric isomerism follow this link.
Beware! It is easy to miss geometric isomers in an exam. Always draw alkenes with the correct 120° bond angles around the C=C bond as shown in the diagrams for the cis and trans isomers above. If you take a short cut and write but-2-ene as CH3CH=CHCH3, you will almost certainly miss the fact that cis and trans forms are possible.
This is a rich source of questions in an exam. You could easily throw away marks if you miss these possibilities.
The Overall Result
Elimination from 2-bromobutane leads to a mixture containing:
- but-1-ene
- cis-but-2-ene (also known as (Z)-but-2-ene)
- trans-but-2-ene (also known as (E)-but-2-ene)
Interactive
Click on the structures to see them in 3D.
Click on the reaction arrows to see an animation of the reaction.
Reproduced with permission from ChemTube3d.com
Where would you like to go now?
To menu of elimination reactions To menu of other types of mechanism To Main Menu