Explaining Electrophilic Addition Reactions Between Unsymmetrical Alkenes and Hydrogen halides
This page guides you through the mechanism for the electrophilic addition of hydrogen halides such as hydrogen bromide to unsymmetrical alkenes like propene.
Important! To make sense of this page, you will need to understand about the structure and stability of carbocations (previously called carbonium ions) and be confident about electrophilic addition to simple alkenes like ethene.
If you aren't sure about either of these things, follow these links first.
You would also find it easier if you first read about the electrophilic addition reactions between hydrogen halides and symmetrical alkenes like ethene, and addition to unsymmetrical alkenes in general. If you have just come from those, ignore these links!
Electrophilic Addition Reactions Involving Hydrogen Bromide
If you want the mechanism for one of the other hydrogen halides, simply replace Br by whatever else you are interested in – F or Cl or I. There is no difference whatsoever in the mechanisms.
You might, however, need to be aware that there is an alternative mechanism involving hydrogen bromide and alkenes if the reaction mixture is impure in the presence of organic peroxides or oxygen from the air.
Note: The different mechanism (a free radical chain reaction – not on UK A-level syllabuses) leads to the hydrogen and bromine adding the opposite way round. For A-level purposes, you don't need to worry about that. However, if you are interested, you will find the free radical addition mechanism by following this link.
Hydrogen bromide as an Electrophile
Hydrogen bromide is chosen as a typical hydrogen halide. Bromine is more electronegative than hydrogen. That means that the bonding pair of electrons is pulled towards the bromine end of the bond, and so the hydrogen bromide molecule is polar.
Note: If you aren't sure about electronegativity and bond polarity follow this link before you read on.
The slightly positive hydrogen atom will be attracted to negative regions in other molecules, and is therefore an electrophile.
Electrophile: A substance with a strong attraction to a negative region in another substance. Electrophiles are either fully positive ions, or the slightly positive end of a polar molecule.
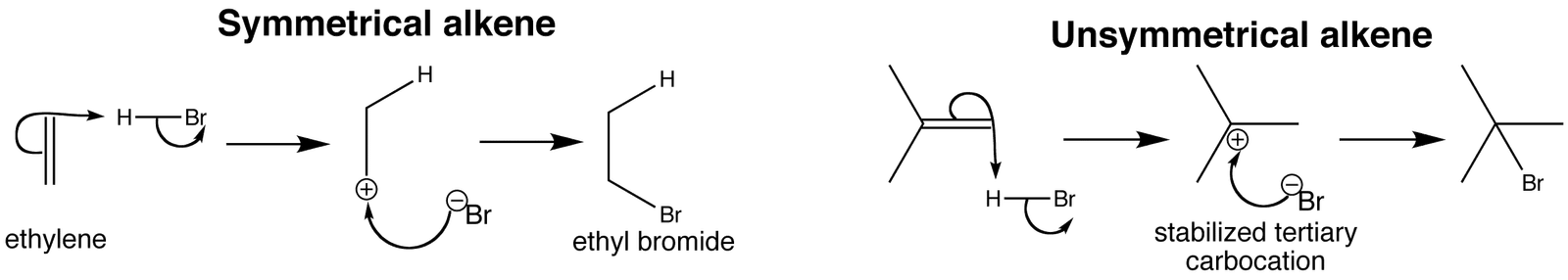
The Reaction of Propene with Hydrogen bromide
The double bond in all alkenes is made up of two different parts. One pair of electrons lies on the line between the two nuclei where you would expect them to be. This is called a sigma bond.
The other pair lies in an orbital above and below the plane of the rest of the molecule, and is called a π bond. The π bond is weaker than a sigma bond and is very vulnerable to attack.
Note: If this isn't fairly obvious to you, you really ought to read the page introducing electrophilic addition before you go on.
As the HBr approaches the π bond, the electrons in that bond are attracted towards the slightly positive hydrogen atom. That repels the electrons in the hydrogen-bromine bond down towards the bromine.
The electron movements continue until a new bond is made between one of the carbon atoms and the hydrogen. The bromine now has both electrons from the H-Br bond, and so is negatively charged as a bromide ion.
The problem is that there are two possible ways that the π bond electrons could move.
They could form a bond between the hydrogen and the left-hand carbon:
or they could form a bond with the right-hand one:
It's the second of these changes that happens more readily. In that case, a secondary carbocation is formed – and that's more energetically stable than the primary one formed in the first possibility.
Because the secondary ion is more energetically stable, it will form more easily and so the reaction needs less activation energy.
Important! If you don't understand about the structure and stability of carbocations (carbonium ions) follow this link.
Activation energy: The minimum energy needed before a reaction will occur.
In this case the activation energy is the energy needed to break the various bonds to make the carbocation and the bromide ion.
Once the ions have been formed, the lone pair on the bromide ion is strongly attracted towards the positive carbon atom. It moves towards it and forms a bond.
Note: There are actually 4 lone pairs around the bromide ion, but we are only interested in the one shown.
That leaves you with the over-all mechanism:
Important! If you've had problems with this page you might find it useful to read about addition to unsymmetrical alkenes in general, and then come back here again.
Interactive
Click on the structures to see them in 3D.
Click on the reaction arrows to see an animation of the reaction.
Reproduced with permission from ChemTube3d.com
Where would you like to go now?
To menu of electrophilic addition reactions To menu of other types of mechanism To Main Menu