Explaining The Reaction Between Symmetrical Alkenes and Bromine
This page guides you through the mechanism for the electrophilic addition of bromine to symmetrical alkenes like ethene or cyclohexene. Unsymmetrical alkenes are covered separately, and you will find a link at the bottom of the page.
The Electrophilic Addition of Bromine to Ethene
The Structure of Ethene
The structure of ethene is shown in the diagramabove. The π bond is an orbital above and below the plane of the rest of the molecule, and relatively exposed to things around it.
Note: If you aren't sure about this, then you should read the page What is electrophilic addition? before you go on.
Bromine as an Electrophile
Since two identical bromine atoms are joined together in the bromine molecule there is no reason why one atom should pull the bonding pair of electrons towards itself – they must be equally electronegative and so there won't be any separation of charge, δ+ or δ-. How, then, can bromine be an electrophile?
Note: If you aren't sure about electronegativity and bond polarity follow this link before you read on.
Equally, if you aren't sure about terms like electrophile, then it really would be a good idea to read the page What is electrophilic addition? before you go on.
In fact, bromine is a very polarisable molecule – in other words, the electrons in the bond are very easily pushed to one end or the other. As the bromine molecule approaches the ethene, the electrons in the π bond tend to repel the electrons in the bromine-bromine bond, leaving the nearer bromine slightly positive and the further one slightly negative.
The bromine molecule therefore acquires an induced dipole which is automatically lined up the right way round for a successful attack on the ethene.
Help! What is an "induced dipole"? A dipole is simply a separation of charge between δ+ at one end and δ- at the other. "Induced" means that it has been created by some external influence (in this case the approach of the π bond), and didn't already exist.
Where it does already exist – as, for example, in HBr – it is called a permanent dipole.
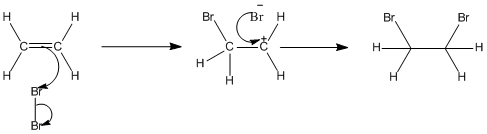
The Simplified Version of the Mechanism
Note: Use this version unless your examiners insist on the more accurate one.
If you've come into this web site from a search engine directly to this page, read the notes on the introductory page to this reaction before you go any further.
The electrons from the π bond move down towards the slightly positive bromine atom.
In the process, the electrons in the Br-Br bond are repelled down until they are entirely on the bottom bromine atom, producing a bromide ion.
Help! If you aren't sure about the use of curly arrows in mechanisms, you must follow this link before you go on.
The ion with a positive charge on the carbon atom is called a carbocation or carbonium ion.
Why is there a positive charge on the carbon atom? The π bond was originally made up of an electron from each of the carbon atoms. Both of those electrons have been used to make a new bond to the bromine. That leaves the right-hand carbon an electron short – hence positively charged.
In the second stage of the mechanism, the lone pair of electrons on the bromide ion is strongly attracted to the positive carbon and moves towards it until a bond is formed.
The overall mechanism is therefore
The More Accurate Version of the Mechanism
Note: Don't learn this unless you have to. There is a real risk of getting confused. If your examiners are happy to accept the simple version, there's no point in making life difficult for yourself.
The reaction starts off just the same as in the simplified version, with the π bond electrons moving down towards the slightly positive bromine atom.
But this time, the top bromine atom becomes attached to both carbon atoms, with the positive charge being found on the bromine rather than on one of the carbons. A bromonium ion is formed.
The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction. It can't be attacked by its original bromide ion because the bromonium ion is completely cluttered up with a positive bromine on that side.
It doesn't matter which of the carbon atoms the bromide ion attacks – the end result would be just the same.
Note: You can't really draw this mechanism tidily in one line because the bromide ion has to be in a different place at the beginning of the second stage than it was at the end of the first stage.
The Electrophilic Addition of Bromine to Cyclohexene
The Simplified Version of the Mechanism
Note: Use this version unless your examiners insist on the more accurate one.
The electrons from the π bond move towards the slightly positive bromine atom.
In the process, the electrons in the bromine-bromine bond are repelled until they are entirely on the right-hand bromine atom, producing a bromide ion.
Exactly as with ethene, a carbocation is formed. The bottom carbon atom lost one of its electrons when the πbond swung towards the bromine.
In the second stage of the mechanism, the lone pair of electrons on the bromide ion is strongly attracted to the positive carbon and moves towards it until a bond is formed.
The overall mechanism is therefore
The Alternative Version of the Mechanism
Note: Don't learn this unless your examiners insist on it. Keep life simple!
The reaction starts off just the same as in the simplified version, with the πbond electrons moving towards the slightly positive bromine atom.
But this time, the left-hand bromine atom becomes attached to both carbon atoms, with the positive charge being found on the bromine rather than on one of the carbons. A bromonium ion is formed.
The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction. It can't be attacked by its original bromide ion because approach from that side is hindered by the positive bromine atom.
It doesn't matter which of the carbon atoms on either end of the original double bond the bromide ion attacks – the end result would be just the same.
Note: Once again, you can't really draw this mechanism tidily in one line because of the need to move the bromide ion.
Interactive
Click on the structures to see them in 3D.
Click on the reaction arrows to see an animation of the reaction.
Reproduced with permission from ChemTube3d.com